Add: 50 Chifeng Road,
Medical Building,
200092,
Shanghai, China
Tel: 021 - 65981041
Fax: 021 - 65981041

Title (s):Professor
E-mail:liweida@tongji.edu.cn
Tel:+86-21-65982037
Address:School of Life Sciences and Technology, Tongji University, Shanghai 200092, China
ORCID:0000-0001-6578-6321
Education
2003 NanKai University B.S.
2009 Peking University Ph.D.
Research Experience
2003-2006 Peking University
2006-2009 National Institute of Biological Sciences, Beijing, China
2010-2015 Harvard Stem Cell Institute, US
2015-present Tongji University, Shanghai, China
Research Interests--Regeneration of human tissues and organs
Trans-differentiation
Stem-Cell and Regenerative Biology
Modeling Diabetes & Wolfram syndrome
Translational Medicine
Biography
Weida Li received his bachelor's degree from Nankai University and Ph. D degree from Peking University. He conducted postdoctoral research at Stem Cell and Regenerative Biology Department of Harvard University where his main research interest focused on developing new therapies for β-cell replacement through stem cells and trans-differentiation technology.
Weida Li received JDRF (Juvenile Diabetes Research Foundation) Fellowship Award and the Chief Scientist of the Stem Cell and Translational Study, Youth Project of National Key Research and Development Program.
Weida Li currently works as a professor at School of Life Sciences and Technology at Tongji University. At same time, he is also an investigator at Translational Medical Center for Stem Cell Therapy and Institute for Regenerative Medicine of Shanghai East Hospital, which is also affiliated to Tongji University.
Research Team:
Li Lab has 20 members, currently including 1 professor, 2 associate professors, 2 research assistant, 13 Ph. D students and 2 students for master degree.
Current Research:
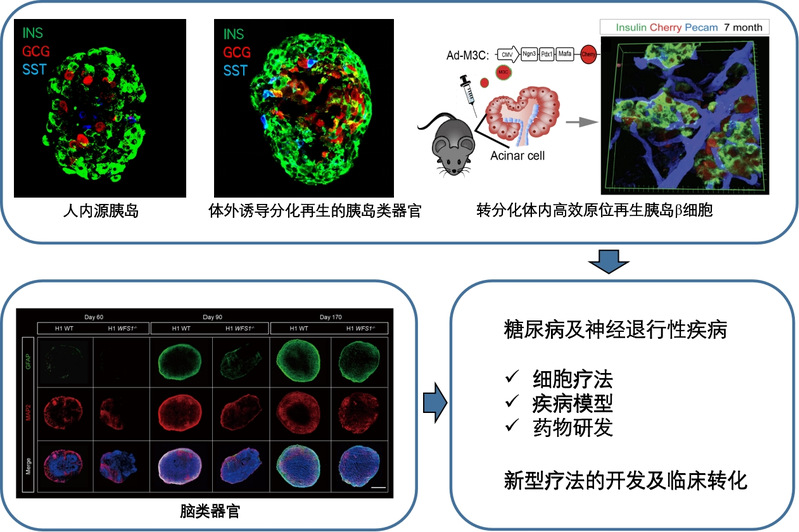
1)Generation functional and diabetes-resistant pancreaticβ-cells with genome editing and human embryonic stem cell differentiation
Human genetics study reveals that SLC30A8 (encoding a zinc transporter, Znt8, mainly expressed in pancreatic islets) loss of function protects human beings from Type 2 diabetes. Nevertheless, the mechanisms underlying this protection, especially in human beings, have not been explored yet. Using CRISPR-Cas9 genome editing technology, loss of function mutation of SLC30A8 was introduced into genome of human embryonic cells (hESCs) that were later differentiated into functional human pancreatic β cells by recapitulating β cell development and maturation process via stepwise differentiation. Our preliminary single cell RNA-seq results revealed that SLC30a8 is mainly involved in β cell maturation process, and further showed that SLC30a8 upregulates insulin secretion pathway in mature β cells. Indeed, we observed that glucose stimulated insulin secretion (GSIS) is significantly enhanced by SLC30A8 loss of function in mature β cells. We also found that SLC30A8 loss of function disrupted zinc transportation into the insulin secretory granules, indicating that there is a correlation of Zinc transportation and insulin secretion upon glucose stimulation. Moreover, β cells harboring SLC30A8 loss of function are resistant to diabetes associated stresses, such as lipotoxicity and glucotoxicity. In summary, our study not only sheds lights on the mechanisms of protection against type 2 diabetes by SLC30A8 loss of function in human pancreatic β cells, but also provides an advanced stem cell-based cell therapy strategy for diabetes.
2) Deciphering the mechanism of reprogramming factors-mediated in vivo transdifferentiation from pancreatic exocrine cells to β-cells
Transdifferentiation of pancreatic exocrine cells to β-cells in vivo by M3 reprogramming factors (Ngn3, Pdx1 and Mafa) holds unprecedented promise for β-cells regeneration in diabetes. Nevertheless, the mechanism remains elusive. In this study, by combination of ATAC-seq (Assay for Transposase-Accessible Chromatin Sequencing) and single cell RNA-seq, we systematically monitor the dynamics of chromatin remodeling and transcriptome remodeling with single cell resolution during the transdifferentiation process. With the aim to decipher the mechanism of M3 reprogramming factors-dependent transdifferentiation, this project identified key factors and pathways as driving forces for chromatin remodeling and investigate their roles in process of transdifferentiation to β-cells from pancreatic exocrine cells and other cell types, enabling us to explore novel regenerative approaches for β-cell replenishment.
3) Disease modeling neurodevelopmental disorder in Wolfram syndrome
Due to limited understanding of pathogenesis in human wolfram syndrome (WS), especially in its neuropathy, there is no effective treatment for WS. Particularly, the causative gene WFS1’s role in human brain neurodevelopment remains elusive. Here, we recapitulated human neurodevelopment and corticogenesis with WFS1 deficiency by integrating the advantages of various human embryonic stem cell (hESCs)-based platforms. 2D neural differentiation was applied to examine WFS1 deficiency with cell autonomous effect. hESCs-derived neural progenitor cells were differentiated into neurons and astrocytes. We found WFS1 deficiency not only renders both neuron progenitor cells vulnerable to cell death, but also causes neuronal developmental disorder, including aberrant gene expressions and abnormalities of neuronal structure. In parallel, decreased GFAP level and disrupted glutamate transportation were observed in WFS1 deficient astrocytes. At same time, to decipher the brain maldevelopment elicited by WFS1 deficiency in spatiotemporal manner, cerebral organoids harboring WFS1 deficiency were generated. WFS1 deficient organoids show reduced organ size, and neuronal cell loss, as well as remarkable retarded astrogenesis. Importantly, co-culture system was applied to simplify the crosstalk affected by WFS1 deficiency between astrocytes and neurons. When co-cultured with neurons, WFS1 deficiency in astrocytes elicits the non-cell-autonomous neurodevelopmental disorder of the neurons derived from wild-type hESCs. On the other hand, astrocytes derived from wild-type hESCs reverse the developmental disorder of neurons harboring the WFS1 deficiency, highlighting the critical and protective role of astrocyte in the WS neuropathy. Altogether, our study reveals WFS1’s essential role in neurogenesis and astrogenesis, and provides novel mechanistic insights into WS neuropathy pathogenesis with a developmental perspective.
Selected Publications:
Liu G*, Li Y*, Li M, Li S, He Q, Liu S, Su Q, Chen X, Xu M, Zhang ZN*, Shao Z#, Li W#. Charting a high-resolution roadmap for regeneration of pancreatic β cells by in vivo transdifferentiation from adult acinar cells. Sci Adv. 2023 May 24;9(21):eadg2183.
Yuan F*, Li Y*, Hu R*, Gong M, Chai M, Ma X, Cha J, Guo P, Yang K, Li M, Xu M, Ma Q, Su Q, Zhang C, Sheng Z, Wu H, Wang Y, Yuan W, Bian S, Shao L, Zhang R, Li K, Shao Z#, Zhang ZN#, Li W#. Modeling disrupted synapse formation in wolfram syndrome using hESCs-derived neural cells and cerebral organoids identifies Riluzole as a therapeutic molecule. Mol Psychiatry. 2023 Feb 7.
Su Y*, Sun J1*, Li X, Qin D, Chen X, Pei Y, Zhang ZN, He Q, Fu Z, Sheng Z, Dong Y, Deng Q, Wang H, Le R#, Gao S#, Li W#. CD47 blocking antibody confers exercise effects by promoting skeletal muscle-specific AMPK activation.Cell Metab. (After review for resubmission)
Ma Q*, Xiao Y*, Xu W*, Wang M, Li S, Yang Z, Xu M, Zhang T, Zhang ZN, Hu R, Su Q, Yuan F, Xiao T, Wang X, He Q, Zhao J, Chen ZJ, Sheng Z, Chai M, Wang H, Shi W, Deng Q#, Cheng X#, Li W#. ZnT8 loss-of-function accelerates functional maturation of hESC-derived β cells and resists metabolic stress in diabetes. Nat Commun. 2022 Jul 16;13(1):4142.
*Research Highlights:
Nature Reviews Endocrinology: “Optimising stem cells for diabetes mellitus therapy.” Science Signaling:“These data indicate the potential of targeting ZnT8 and zinc regulation in engineering more functionally robust SC-β cells toward the treatment of insulin-dependent diabetes mellitus.”
Ø Baek AE. Stemming metabolic stress. Sci Signal. 2022 Jul 26;15(744):eade0564.
Ø Starling S. Optimising stem cells for diabetes mellitus therapy. Nat Rev Endocrinol. 2022 Oct;18(10):588.
* Authorized Patent:CN111411072A
Sun J*, Su Y*, Xu Y, Qin D, He Q, Qiu H, Zhuo J, Li W#. CD36 deficiency inhibits proliferation by cell cycle control in skeletal muscle cells. Front Physiol. 2022 Aug 30;13:947325.
Liu G*, Li Y*, Zhang T*, Li M*, Li S, He Q, Liu S, Xu M, Xiao T, Shao Z#, Shi W#, Li W#. Single-cell RNA Sequencing Reveals Sexually Dimorphic Transcriptome and Type 2 Diabetes Genes in Mouse Islet β Cells. Genomics Proteomics Bioinformatics. 2021 Jun;19(3):408-422.
Wang X*, Zhou R*, Xiong Y, Zhou L, Yan X, Wang M, Li F, Xie C, Zhang Y, Huang Z, Ding C, Shi K, Li W, Liu Y, Cao Z, Zhang ZN, Zhou S, Chen C, Zhang Y#, Chen L#, Wang Y#. Sequential fate-switches in stem-like cells drive the tumorigenic trajectory from human neural stem cells to malignant glioma. Cell Res. 2021 Jun;31(6):684-702.
Gong L, Cao L, Shen Z, Shao L, Gao S, Zhang C#, Lu J#, Li W#. Materials for Neural Differentiation, Trans-Differentiation, and Modeling of Neurological Disease. Adv Mater. 2018 Apr;30(17):e1705684.
Cao L, Hu R, Xu T, Zhang ZN, Li W, Lu J. Characterization of Induced Pluripotent Stem Cell-derived Human Serotonergic Neurons. Front Cell Neurosci. 2017 May 8;11:131.
Lv S, Li J, Qiu X, Li W, Zhang C, Zhang ZN#, Luan B#. A negative feedback loop of ICER and NF-κB regulates TLR signaling in innate immune responses. Cell Death Differ. 2017 Mar;24(3):492-499.
Lv S, Qiu X, Li J, Liang J, Li W, Zhang C, Zhang ZN, Luan B. Glucagon-induced extracellular cAMP regulates hepatic lipid metabolism. J Endocrinol. 2017 Aug;234(2):73-87.
Cavelti-Weder C*, Li W*, Zumsteg A, Stemann-Andersen M, Zhang Y, Yamada T, Wang M, Lu J, Jermendy A, Bee YM, Bonner-Weir S, Weir GC, Zhou Q. Hyperglycaemia attenuates in vivo reprogramming of pancreatic exocrine cells to beta cells in mice. Diabetologia. 2016 Mar;59(3):522-32.
Lv S, Qiu X, Li J, Li W, Zhang C, Zhang ZN, Luan B. Suppression of CRTC2-mediated hepatic gluconeogenesis by TRAF6 contributes to hypoglycemia in septic shock. Cell Discov. 2016 Dec 13;2:16046.
Cavelti-Weder C, Li W, Zumsteg A, Stemann M, Yamada T, Bonner-Weir S, Weir G, Zhou Q. Direct Reprogramming for Pancreatic Beta-Cells Using Key Developmental Genes. Curr Pathobiol Rep. 2015 Mar 1;3(1):57-65.
Yamada T, Cavelti-Weder C, Caballero F, Lysy PA, Guo L, Sharma A, Li W, Zhou Q, Bonner-Weir S, Weir GC. Reprogramming Mouse Cells With a Pancreatic Duct Phenotype to Insulin-Producing β-Like Cells. Endocrinology. 2015 Jun;156(6):2029-38.
Li W*, Cavelti-Weder C*, Zhang Y*, Clement K, Donovan S, Gonzalez G, Zhu J, Stemann M, Xu K, Hashimoto T, Yamada T, Nakanishi M, Zhang Y, Zeng S, Gifford D, Meissner A, Weir G, Zhou Q. Long-term persistence and development of induced pancreatic beta cells generated by lineage conversion of acinar cells. Nat Biotechnol. 2014 Dec;32(12):1223-30.
Li W*, Nakanishi M*, Zumsteg A, Shear M, Wright C, Melton DA, Zhou Q. In vivo reprogramming of pancreatic acinar cells to three islet endocrine subtypes. Elife. 2014 Jan 1;3:e01846.
Wang X, Li W, Zhao D, Liu B, Shi Y, Chen B, Yang H, Guo P, Geng X, Shang Z, Peden E, Kage-Nakadai E, Mitani S, Xue D. Caenorhabditis elegans transthyretin-like protein TTR-52 mediates recognition of apoptotic cells by the CED-1 phagocyte receptor. Nat Cell Biol. 2010 Jul;12(7):655-64.
Li W, Zou W, Zhao D, Yan J, Zhu Z, Lu J, Wang X. C. elegans Rab GTPase activating protein TBC-2 promotes cell corpse degradation by regulating the small GTPase RAB-5. Development. 2009 Jul;136(14):2445-55.
Lu Q*, Zhang Y*, Hu T*, Guo P, Li W, Wang X. C. elegans Rab GTPase 2 is required for the degradation of apoptotic cells. Development. 2008 Mar;135(6):1069-80.
Cronican JJ, Beier KT, Davis TN, Tseng JC, Li W, Thompson DB, Shih AF, May EM, Cepko CL, Kung AL, Zhou Q, Liu DR. A class of human proteins that deliver functional proteins into mammalian cells in vitro and in vivo. Chem Biol. 2011 Jul 29;18(7):833-8.
Zou W*, Lu Q*, Zhao D, Li W, Mapes J, Xie Y, Wang X. Caenorhabditis elegans myotubularin MTM-1 negatively regulates the engulfment of apoptotic cells. PLoS Genet. 2009 Oct;5(10):e1000679.