Osteoarthritis (OA) is a common degenerative joint disease affecting approximately 240 million people worldwide. Its incidence increases significantly with age. Major symptoms of OA include joint space narrowing, cartilage erosion, subchondral bone thickening, synovial inflammation, chronic pain, joint deformity, and restricted mobility, often leading to end-stage disability. Current pharmacological treatments—primarily oral non-steroidal anti-inflammatory drugs and intra-articular corticosteroid injections—can alleviate joint pain and inflammation but fail to repair cartilage damage. End-stage OA can only be managed through joint replacement surgery to improve patients' quality of life. Matrix-associated Autologous Chondrocyte Implantation (MACI) is a clinical procedure used to repair cartilage defects. However, the therapeutic efficacy of MACI is significantly limited by proliferative capacity of autologous chondrocytes in vitro, which declines sharply with age. As a result, this therapy is restricted in the repair area size and is only applicable to patients aged 18-55, greatly restricting its broader clinical application.
Professor Rui Yue’s team from School of Life Sciences and Technology at Tongji University, in collaboration with other researchers, has made significant progress in understanding the maintenance and regeneration of articular cartilage. Their findings, recently published in the top life science journal Cell, identified a population of Procr⁺ chondroprogenitors that are sensitive to mechanical stimuli. These cells are essential not only for maintaining joint cartilage homeostasis but also for promoting cartilage regeneration after injury, making them a promising next-generation seed cell for treating degenerative joint diseases.
The researchers found that Procr⁺ chondroprogenitor cells are primarily distributed in the superficial zone of cartilage subjected to higher mechanical loads, such as the tibial plateau and meniscus of the knee joint. These cells possess the ability to differentiate into middle/deep zone chondrocytes, thereby closely regulating postnatal articular cartilage homeostasis. The study revealed that increased mechanical stimulation significantly boosts the number of Procr⁺ cells in articular cartilage and the meniscus, while reduced mechanical stimulation significantly decreases their numbers, indicating that mechanical stimuli tightly regulate the distribution and abundance of Procr⁺ cells in articular cartilage.
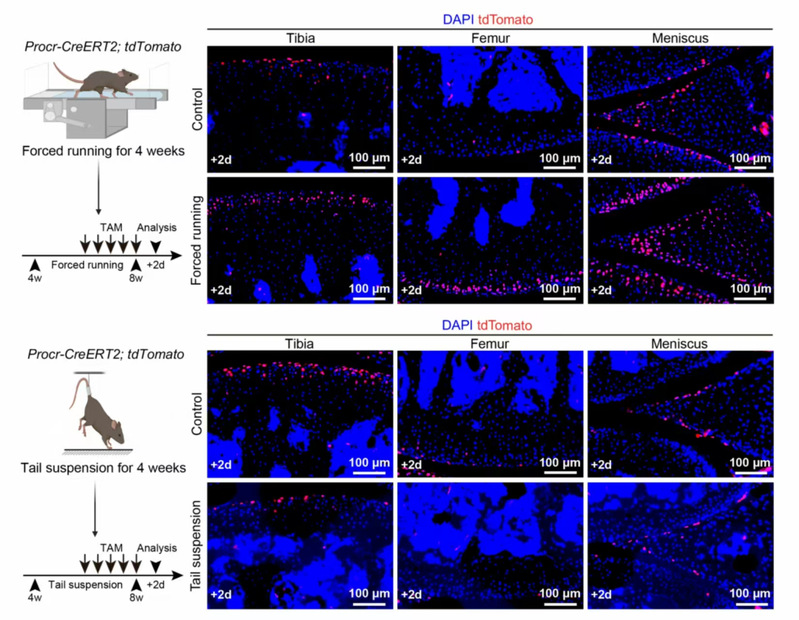
Fig. 1 Mechanical stimulation regulates the distribution and frequency of Procr+ cells in the articular cartilage and meniscus
The researchers also discovered that compared to healthy control mice, OA model mice exhibited a significant increase in chondrocytes derived from Procr⁺ cells in the tibia, femur, and meniscus. Importantly, ablation of Procr⁺ cells led to exacerbated joint damage in OA mice, indicating that these cells help delay OA progression through cartilage regeneration. Single-cell RNA-sequencing analysis of superficial zone cells revealed that the transcriptional regulatory network of the mechanosensitive transcription factor Klf2 was activated in the OA group. Klf2 mediates mechanical signal transduction via Piezo1/2 and Trpv4 ion channels, with Piezo1 being most highly expressed in superficial zone cells. Notably, deletion of Piezo1 resulted in a significant reduction of progeny chondrocytes derived from Procr⁺ cells in OA mice and more severe OA symptoms.
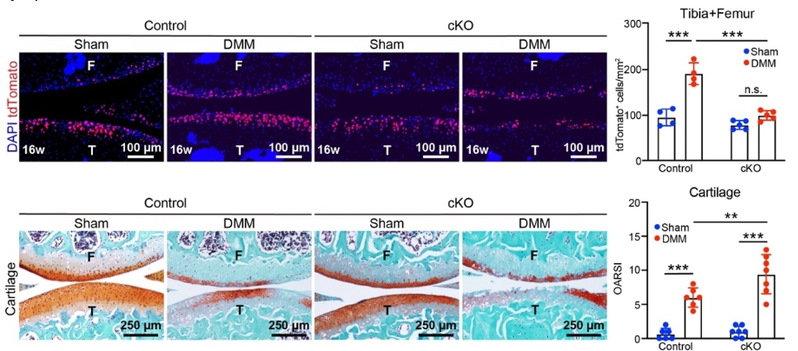
Fig. 2 Genetic deletion of Piezo1 impaired Procr+ cell activation and articular cartilage regeneration
To assess the regenerative potential of Procr⁺ cells, researchers isolated Procr⁺ cells and performed 2D expansion, 3D sphere culture, and in vivo transplantation experiments. Procr⁺ cells demonstrated robust clonogenicity and chondrogenic differentiation potential. In both ectopic renal subcapsular transplantation and orthotopic cartilage defect transplantation, Procr⁺ cells exhibited superior cartilage regeneration ability. Importantly, similar PROCR⁺ cells were isolated from articular cartilage samples of both young and old human donors. In vivo transplantation showed that these human PROCR⁺ cells possess more robust cartilage repair capabilities as compared to mature chondrocytes.
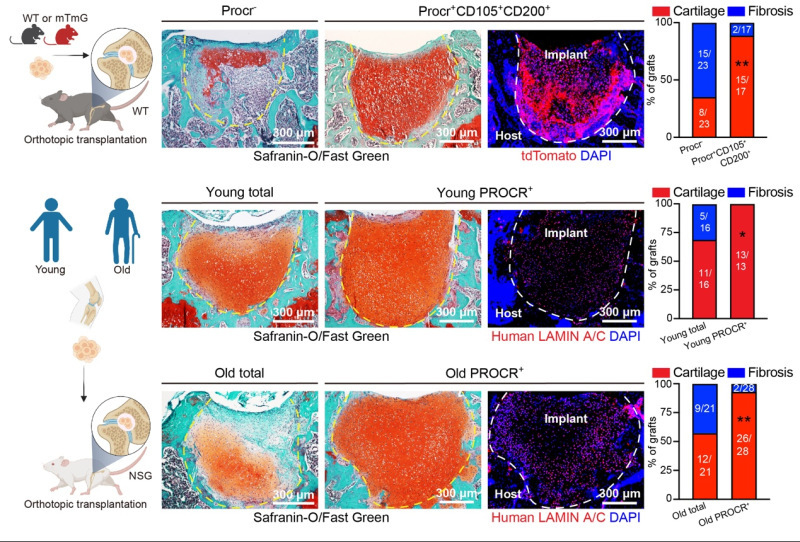
Fig. 3 In vivo transplantation of PROCR⁺ cells for cartilage repair
In conclusion, this study identified mechanosensitive Procr⁺ chondroprogenitors in the superficial zone of mouse knee joint cartilage and meniscus, which play a fundamental role in maintaining cartilage homeostasis and promoting regeneration via Piezo1-mediated mechanotransduction. Furthermore, human PROCR⁺ cells were shown to be a potential source for cell therapy to efficiently regenerate articular cartilage.
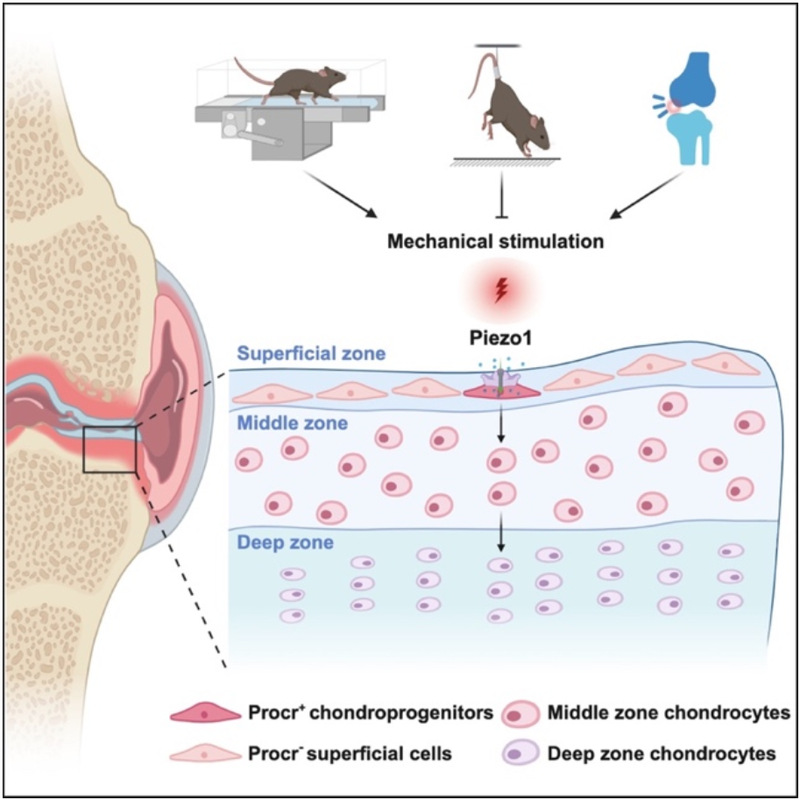
Fig. 4 Procr+ chondroprogenitors sense mechanical stimuli to govern articular cartilage maintenance and regeneration
Professor Rui Yue from the School of Life Sciences and Technology at Tongji University and Professor Weiguo Zou from Hainan Medical University are the co-corresponding authors of this study. Qiaoling Zhu, a Ph.D. candidate at the School of Life Sciences and Technology, Tongji University; Dr. Feng Yin, Associate Professor and Director of the Department of Joint and Bone Diseases at Tongji University-affiliated East Hospital; and Jiachen Qin and Wanyu Shi, Master's students at the School of Life Sciences and Technology, are co-first authors of this paper. This work was supported by grants from the National Key R&D Program of China, National Natural Science Foundation of China, Peak Disciplines (Type IV) of Institutions of Higher Learning in Shanghai, Shanghai Pilot Program for Basic Research, and Fundamental Research Funds for the Central Universities.
Paper Link: https://www.cell.com/cell/fulltext/S0092-8674(25)00738-X