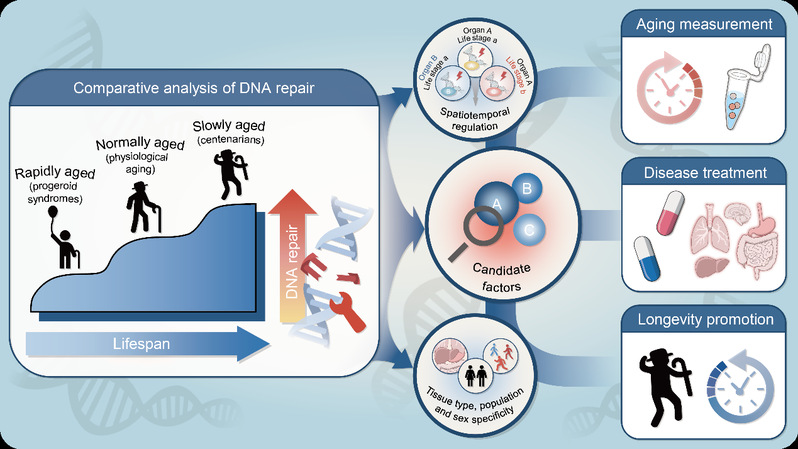
DNA carries the genetic information essential for life. However, it faces constant threats from internal and external factors, leading to various types of damage that compromise genome stability. This instability drives aging and related diseases. Current research on DNA repair and aging heavily relies on animal models, particularly mice, due to the scarcity of human samples. Yet, it remains unclear whether the changes in repair dynamics during mouse aging, and their biological consequences, fully reflect human aging processes. Consequently, elucidating DNA repair mechanisms linked to human aging and longevity remains a critical future research direction. Humans exhibit remarkable diversity in aging rates and lifespans, ranging from progeria (accelerated aging) to exceptional longevity (slow aging), with nearly a 10-fold difference in lifespan. This spectrum provides a powerful framework for comparative studies of DNA repair mechanisms.
A recent review published in Ageing Research Reviews by a research team from School of Life Sciences and Technology, Tongji University and Shanghai First Maternity and Infant Hospital offers a comprehensive summary of the current state of knowledge in the field of DNA repair and aging. The authors propose a novel comparative biology approach to investigate DNA repair regulation across the human lifespan. This approach promises to facilitate a more profound comprehension of the intricate regulatory mechanisms underpinning DNA repair, along with their spatial and temporal variability during the process of aging. Moreover, it offers a distinctive framework for uncovering the mechanisms underlying longevity and promoting healthy aging.

The first part of this review establishes that the balance between DNA damage and repair becomes progressively disrupted during physiological aging. Extensive research across diverse animal models demonstrates a consistent age-related decline in the efficiency of multiple DNA repair pathways. This decline results in the progressive accumulation of DNA damage. Human studies corroborate this phenomenon, revealing a similar decrease in overall repair capacity with advancing age. Furthermore, different tissues exhibit distinct, tissue-specific alterations in repair capability during aging, governed by unique underlying molecular mechanisms. Critically, DNA damage and repair do not occur in isolation. They interact and intertwine with other core aging hallmarks—including protein homeostasis imbalance, epigenetic alterations, telomere dysfunction, and disabled autophagy—to collectively drive the aging process.
Part two centers on progeria research. Studies of progeroid syndromes not only inform therapeutic development for rare diseases but also offer critical insights into the relationship between DNA repair and aging. Multiple forms of progeria have been directly linked to defective DNA repair mechanisms. Notably, patients exhibit tissue-specific and developmental-stage-specific accelerated aging phenotypes, indicating that DNA repair factors function with spatiotemporal specificity and that tissues or developmental stages vary in their sensitivity to repair deficiencies. Conversely, not all mutations in DNA repair factors induce premature aging, and some repair proteins possess moonlighting functions beyond their core repair roles. These findings underscore that progeria pathogenesis is highly complex, involving crosstalk between DNA repair and other pathways, with specific mechanisms yet to be fully defined.
The third section reviews research on longevity populations. Substantial evidence indicates that maintaining genomic stability is critical for achieving both longevity and healthy aging. Synthesizing findings across diverse cohorts, the review highlights that these populations exhibit a distinctive capacity to preserve efficient DNA repair mechanisms, thereby safeguarding genomic integrity. This capacity is underpinned by multi-level regulatory processes, spanning genetic, epigenetic, and metabolic adaptations. Critically, the study identifies specific DNA repair gene variants significantly associated with extended lifespan, providing a foundation for future interventions targeting DNA repair pathways to promote healthy longevity.
Although significant progress has been made at the intersection of DNA repair, aging and longevity in recent years, the review concludes by pointing out that the field still faces many challenges. Future research may focus on: (1) Elucidating the tissue-specific and temporal regulation of DNA repair; (2) Investigating gender differences and population variability in DNA repair capacity; (3) Developing aging markers and clocks based on DNA damage and repair signatures; (4) Exploring the potential of targeted DNA repair activation to promote longevity, including clinical translation pathways and associated risk assessments.
In summary, this review systematically establishes the pivotal role of DNA repair in regulating human aging and longevity. It highlights comparative biology approaches as a powerful strategy for deciphering the fine-tuned regulation of DNA repair mechanisms and advancing healthy aging. Ultimately, it underscores the promising prospect of achieving successful aging through targeting DNA repair.
Assistant Professor Yu Chen and Professor Zhiyong Mao from the School of Life Sciences and Technology, Tongji University, and Shanghai First Maternity and Infant Hospital are the co-corresponding authors of the paper. Yunjia Tang, an undergraduate student from the School of Life Sciences and Technology, Tongji University, is the first author of the paper. Dekai Zhang, an undergraduate student, and Kaiyan Wang, a master's candidate, also contributed to the research. This work was supported by the National Natural Science Foundation of China, the Shanghai Science and Technology Commission, and the Fundamental Research Funds for the Central Universities.
Paper Link:
Decoding DNA repair regulation across human lifespan variability
https://doi.org/10.1016/j.arr.2025.102833