RNA modification regulates nuclear apparent modification and
early development by reversing the transposon
On May 8, 2022, the research team headed by Professor GAO Shaorong and GAO Yawei from the School of Life Science and Technology of Tongji University published an online article entitled “FTO mediates LINE1 m6A demethylation and chromatin regulation in mESCs and mouse development” in Science in collaboration with Professor HE Chuan from the University of Chicago. It is discovered that in mouse embryonic stem cells (mESCs), mouse and human tissues, mouse oocytes and their early development, FTO can regulate chromatin related RNA, affect chromatin opening and histone modification to affect the proliferation and differentiation of mESCs and the normal development of early embryos. This research is of great significance to analyze the biological functions in the dynamic regulation of RNA m6A modification in mammals and their development.
Paper published in Science
N6-methyladenine (m6A) is the most common modification in eukaryotic mRNA which has attracted extensive research. It is regulated by writing protein, erasing protein and realizes its function through reading protein. M6A plays a vital role in the regulation of mRNA and is involved in the regulation of splicing, transport, degradation, translation and phase separation. It affects life processes such as ontogeny and differentiation, and has been found to play an important role in a variety of diseases, especially cancer and immune diseases. However, little in-depth research has been conducted on the role of m6A erasure protein mediated dynamic demethylation in this regulation and its function in mammalian development.
FTO is the first identified RNA demethylase which is related to mammalian development and human diseases, and is involved in the regulation of carcinogenesis and development. The research headed by Professor HE from the University of Chicago and Professor GAO Yawei / GAO Shaorong from Tongji University, discovered that the proportion of homozygous knockout offspring produced by FTO knockout heterozygous mice was low, and homozygous knockout female mice could not produce healthy and viable offspring, suggesting that the FTO protein of mice may be significant for reproductive development. The team established the FTO knockout embryonic stem cell line (FTO -/- MESC) with the help of the embryos produced by FTO knockout heterozygous mice. The researchers found out that FTO knockout will increase the m6A modification level of chromatin related RNA (caRNA) in mESC, and lead to the decrease of transcriptional activity and chromatin opening at the genomic level. These changes will lead to cell proliferation defects and damage the differentiation potential of cells in vivo and in vitro.
Their research analysis of various omics technologies showed that FTO knockout would affect the accumulation of m6A modification on the RNA of retrotransposon LINE1 in the genome, accelerate the degradation of RNA and reduce the transcriptional activity, thus reducing the RNA abundance of LINE1. Moreover, the researchers also found that the decrease of LINE1 RNA affected the transcriptional silencing of LINE1 to downstream target genes (including 2C gene and retrotransposon). At the same time, with the decrease of LINE1 abundance, the coding genes near LINE1 will show an obvious decline in transcript abundance and transcriptional activity, including a large number of important genes related to stem cell differentiation and ontogeny.
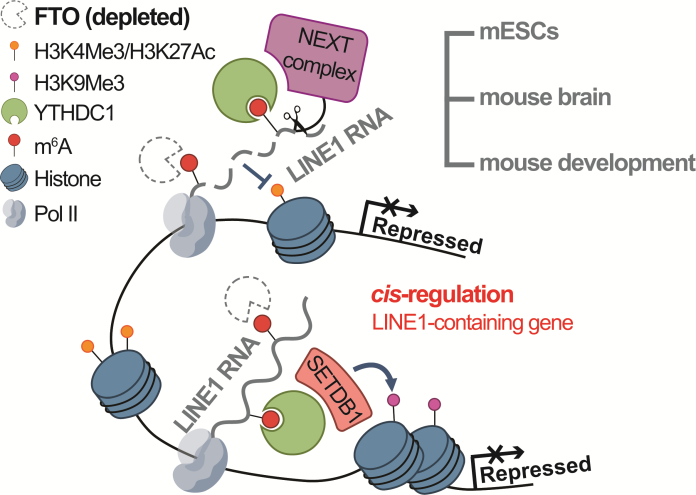
Figure 1 FTO regulates LINE1 RNA, chromatin status nearby
and cis regulation of LINE1 containing gene
Tongji's research team proved that the loss of FTO protein would affect the differentiation and maturation of oocytes, and such defective oocytes would have serious implantation and development problems in the case of complete loss of FTO protein by making use of their own modifications in embryonic development. The team successfully proved that FTO was involved in the regulation of LINE1 RNA in oocyte development and early embryos, and will also be involved in the opening of chromatin in the nucleus, the transcriptional activity regulation of Line1 downstream target genes (including 2C gene and retrotransposon), and the coding genes near LINE1. The team demonstrated by using the LINE1 RNA site-specific m6A erasure system that LINE1 RNA abundance, intranuclear chromatin development and coding gene abnormalities were indeed caused by the accumulation of m6A modification on LINE1.
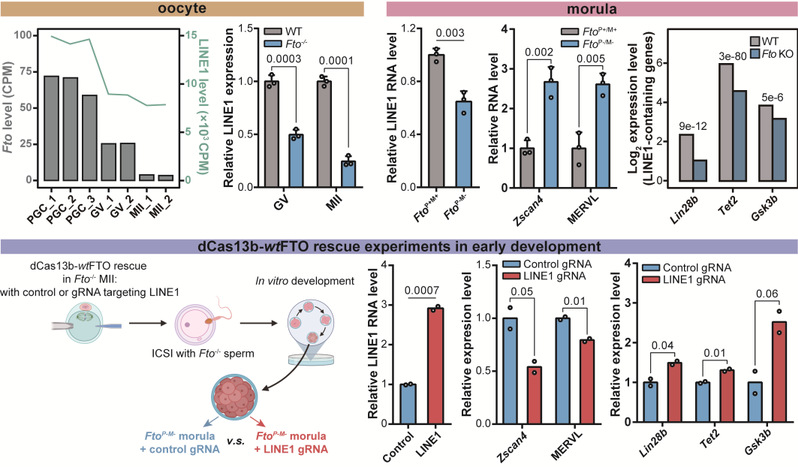
Figure 2. FTO in the regulation of LINE1 RNA and downstream genes
in egg development and early embryos
According to Professor GAO Shaorong, the research identified LINE1 RNA m6A as the main functional substrate of FTO in mESCs and during mouse development. It not only described the new mechanism of FTO in realizing nuclear chromatin apparent modification and LINE1 downstream and enriched gene expression by regulating the apparent modification of LINE1 RNA in cell lines, but also proved this mechanism in vivo development. This research provides a new perspective for further analysis of the molecular regulation mechanism of life process.
In addition, another research team headed by Professor JIA Guifang / HE Chuan / SONG Baoan used overexpression of FTO to increase the yield of rice and potato by 50%, and its molecular mechanism may also be related to m6A demethylation and chromatin regulation of repeat RNA in plants, suggesting that m6A demethylation of repeat RNA may have important regulatory functions on the apparent systems in different species systems.
Dr. WEI Jiangbo and Dr. YU Xianbin of the University of Chicago, and doctoral students YANG Lei and LIU Xuelian of Tongji University are the co-authors of this paper while Professor HE Chuan of the University of Chicago, Professor GAO Shaorong and Professor GAO Yawei of Tongji University corresponding authors. Professor LI Xuekun of Zhejiang University, HUANG Boxian, associate researcher of Nanjing Medical University and LIU Jun, researcher of Peking University also participated in this research. The research was supported by the Ministry of Science and Technology, the National Natural Science Foundation of China and the Shanghai Science and Technology Commission.